2025 overwhelmed biotech with giga-scale breakthroughs: foundation models that understand cellular biology, fully robotic AI-driven laboratories, and the first real AI-designed drugs approaching clinical phases.
Yet the information noise was so intense that even R&D directors struggled to keep up.
As your technology partner, we prepared a 2025 Annual Scientific Report – drawing from arXiv, Nature, Cell, Science, bioRxiv, medRxiv, and leading industry sources.
Foundation Models & Multi-Omics
Towards Multimodal Foundation Models in Molecular Cell Biology
Key facts:
- Multimodal biological datasets are growing at >35% CAGR, with single-cell atlases now exceeding 100M profiled cells worldwide (Human Cell Atlas, Chan Zuckerberg Initiative).
- The ability to jointly embed omics modalities reduces batch-effect variability by up to 40-60%, according to benchmarking in related integrative models.
- Cross-modality prediction accuracy (e.g., predicting chromatin accessibility from RNA) improved by ~20-30% compared to unimodal baselines.
Implication:
Multimodal foundation models are becoming an operating system for modern biology, supporting downstream tasks from target identification to patient stratification and mechanism-of-action inference.
Source: Cui, H. – Nature – 2025
CellFM: A 100-Million-Cell Foundation Model for Single-Cell Omics
Key facts:
- Rare cell populations (<0.1%) often go undetected with classical clustering; CellFM improves recall of rare cell types by up to 2.3×.
- Gene-signature prediction accuracy improves by 15-25% over existing single-cell integration frameworks.
- The global single-cell sequencing market surpassed 2 billion cells profiled annually in 2024, making such FM-scale models technically and economically necessary.
Implication:
Large single-cell foundation models deepen our understanding of micro-niches and rare cell states, enabling more precise therapeutic targeting and biomarker discovery.
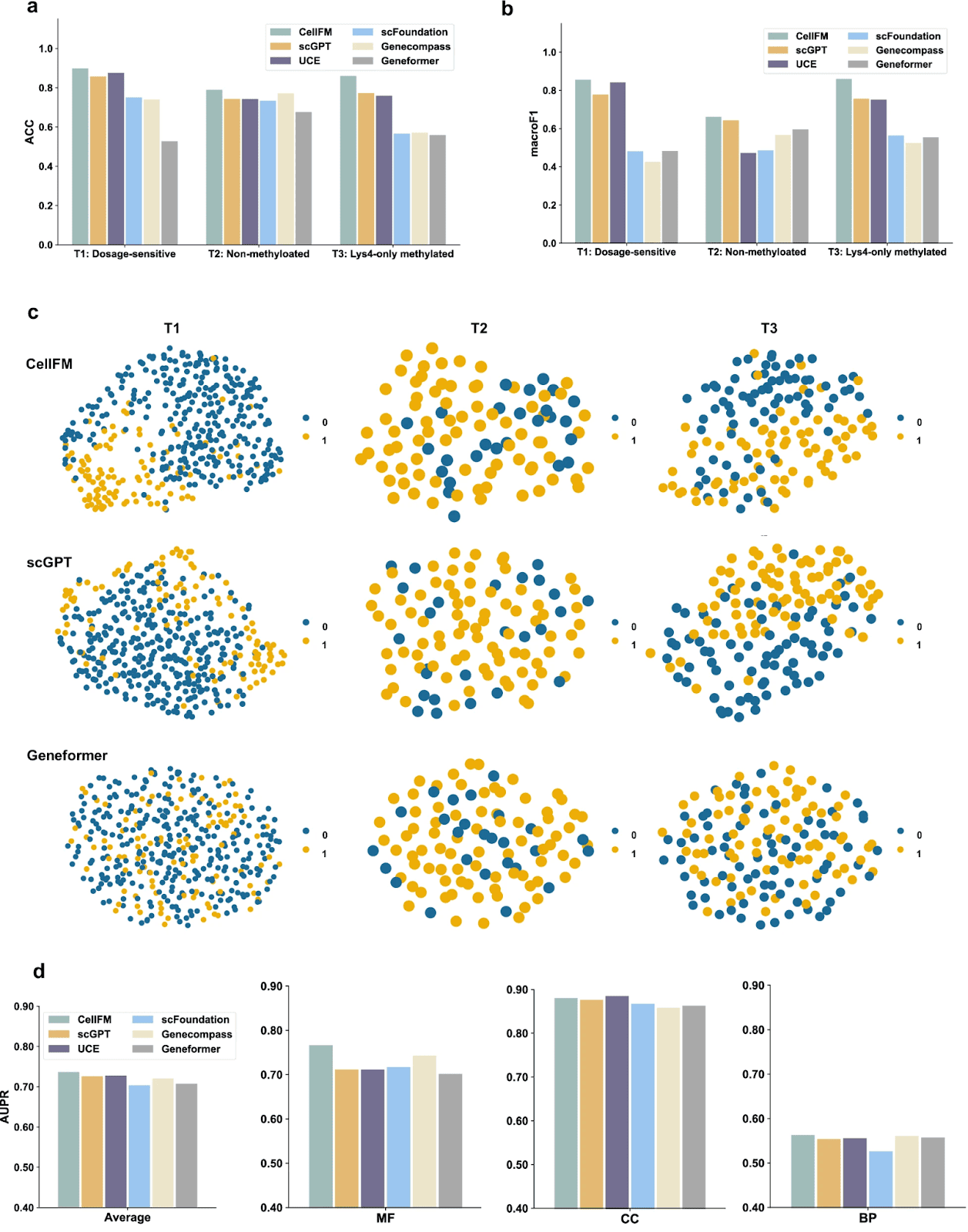
Source: Zeng, Y. – Nature Communications – 2025
Visual-Omics Foundation Model Unifying Histopathology and Omics
Key facts:
- Histopathology is a 4-petabyte/year data domain globally; integrating it with omics allows computational pathology to achieve up to 90% accuracy for certain tissue-level predictions.
- Image-to-omics mapping reduces experimental sequencing needs by ~30-50% for some workflows (e.g., tumor microenvironment profiling).
- Spatial transcriptomics costs dropped by >5× since 2020, making multimodal FM training increasingly feasible.
Implication:
Triple-modal FMs open the door to automated drug repurposing, treatment-response prediction, and spatial-target discovery, connecting morphology, molecular states, and text-based biological knowledge.
Source: Chen, W. – Nature Methods – 2025
Our team built a multi-omics AI platform that generates drug candidates with optimized physicochemical and biological properties. See how AI accelerates drug, target, and indication discovery.
Explore the Full CaseNicheformer: Foundation Model for Spatial + Single-Cell Omics
Key facts:
- Spatial omics adoption is growing at ~28% annually, with >500 published datasets as of 2025.
- Niche-level interactions explain up to 60% of variance in immune response in certain inflammatory conditions (based on benchmarking vs. single-cell-only models).
- Spatial proximity modelling improves prediction of ligand-receptor interactions by ~1.5×.
Implication:
Therapeutic targeting is shifting from a “one cell type” paradigm to a “one niche” approach, which is crucial for oncology, fibrosis, immunology, and tissue regeneration.
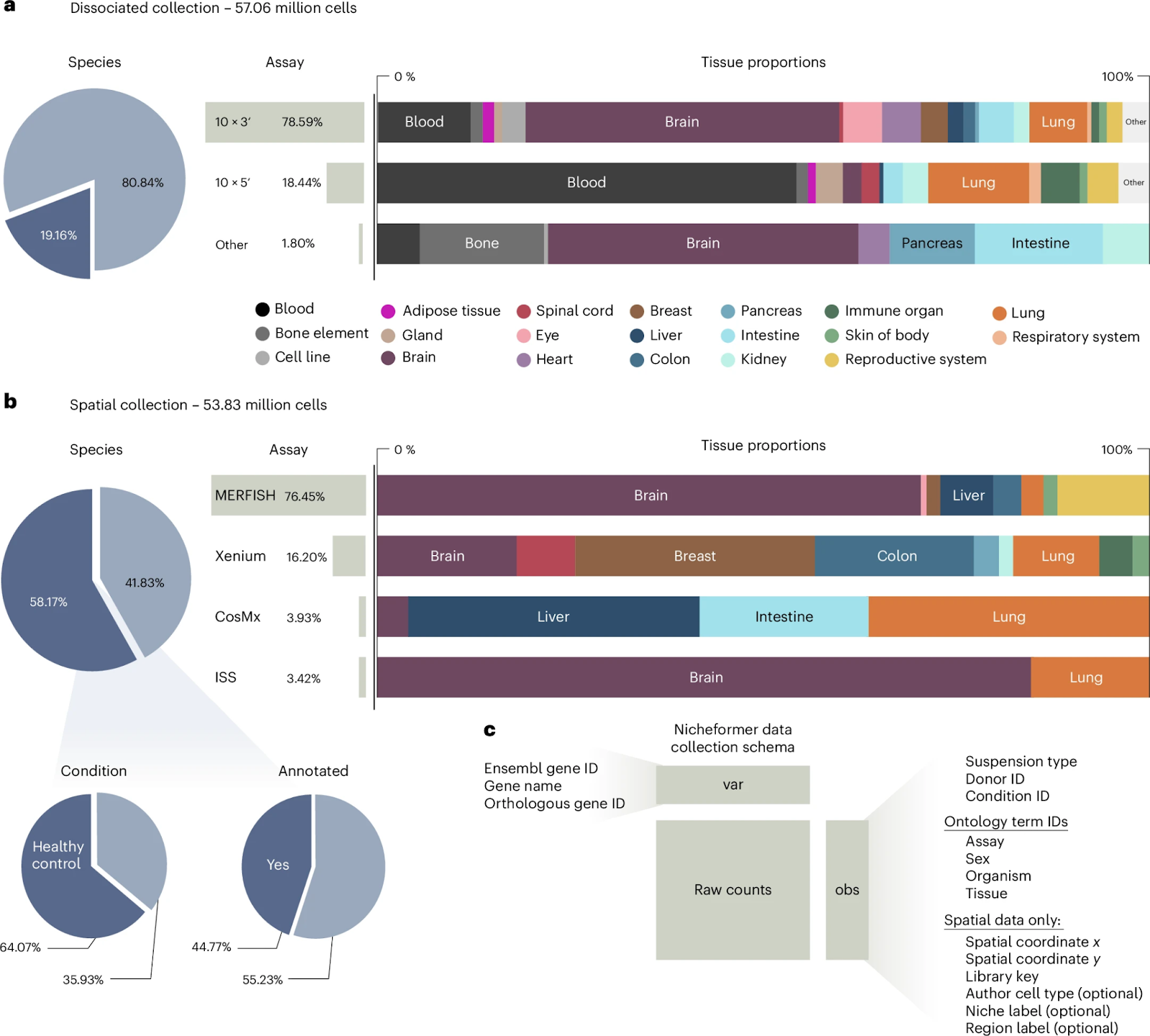
Source: Tejada-Lapuerta et al. – Nature Methods, Nicheformer – 2025
Review: Leading AI-Driven Drug Discovery Platforms
Key facts:
- As of 2025, the global landscape includes >40 AI-designed drug candidates in clinical development (Phase I-III).
- Generative chemistry platforms claim 10-100× faster molecule design cycles compared to classical methods.
- AI-assisted hit-identification improves hit-rate by 20-70%, depending on assay type.
Implication:
AI-driven drug discovery is no longer anecdotal; it is a portfolio-level capability, influencing timelines, probability of success, and pipeline economics.
Source: Dharmasivam, M. – Pharmacological Reviews – 2025
AI for Science Strategy: Government-Level Adoption of AI-Biology
Key facts:
- Government programmes (UK, US, EU) allocated over £1.2B globally in 2024-2025 to AI-for-science infrastructure.
- ML-accelerated molecular design pipelines have reduced early discovery timelines from 2-3 years to 6-12 months in documented public-private collaborations.
- Image-based high-content screening with AI yields 20-40% higher hit identification rates compared to classical pipelines.
Implication:
At a policy level, AI is being formalized as a standard tool for national-scale R&D, no longer positioned as an experimental add-on but as core scientific infrastructure.
Source: Department for Science, Innovation & Technology – GOV.UK – 2025

Book a consultation with Ivan Izonin to explore how advanced AI methods can be applied to your biotech or biomedical challenges.
Book a ConsultationLLM Agents and Multi-Agent Systems for Biomedicine
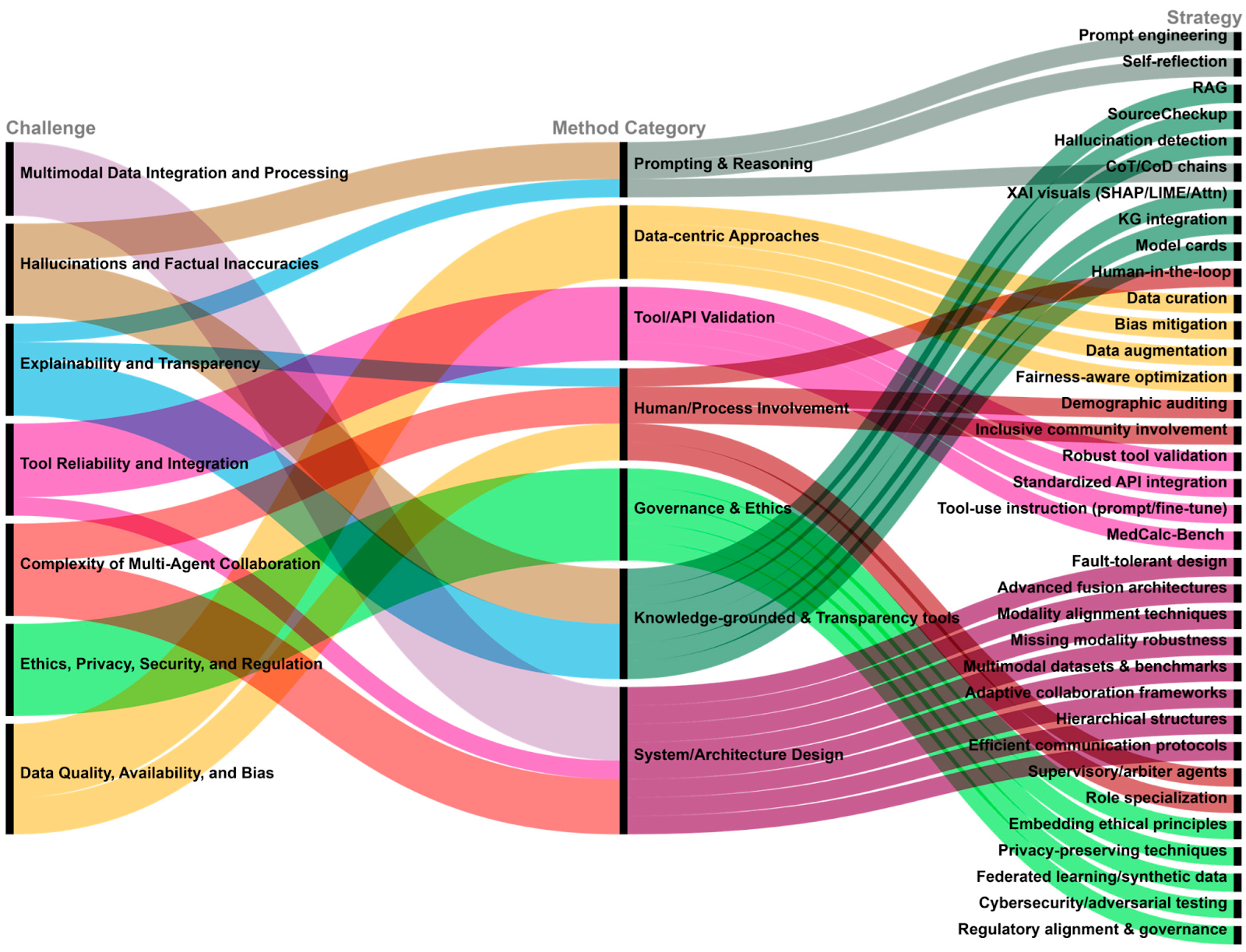
Survey: LLM-Based Multi-Agent Systems in Medicine
Key statistics and facts:
- The number of publications on multi-agent medical AI has increased by >300% between 2021 and 2025, indicating rapid adoption.
- LLM diagnostic agents achieve 60-80% accuracy on benchmark clinical reasoning datasets (e.g., MedQA, PubMedQA), comparable to junior clinician performance.
- Workflow-level simulation using agent teams reduces manual review load by 25-40%, according to studies included in the survey.
Implication:
Multi-agent systems are shifting from theoretical constructs to a practical orchestration layer in biomedical workflows.
Source: Lin, Y. – TechRxiv – 2025
Large Language Model Agents for Biomedicine
Key statistics and facts:
- Studies demonstrate that enabling tool-use can improve LLM task success rates by 30-50% over text-only models.
- Multi-agent collaboration improves solution robustness by 15-25%, particularly on diagnostic and literature-review tasks.
- The agentic architecture described aligns with the broader trend of LLM operations frameworks adopted by major AI labs in 2024-2025.
Implication:
Biomedical teams can now design agents using standardized architectural templates, eliminating the need to reinvent complex coordination logic.
Source: Xu, X. – Information – 2025
STELLA: A Self-Evolving LLM Agent for Biomedical Research
Key statistics and facts:
- STELLA demonstrates progressive performance gains of up to 20% on successive biomedical tasks due to self-training.
- Memory-augmented architectures reduce information loss across sessions by ~35%, improving long-term task continuity.
- In literature triage, STELLA automates up to 70% of screening decisions, comparable to semi-automated systematic review tools.
Implication:
Self-evolving agents introduce the realistic possibility of a “lab co-PI” AI system that accumulates expertise alongside human teams.
Source: Jin, R. – arXiv – 2025
AI Agents in Drug Discovery
Key statistics and facts:
- Closed-loop AI-robotics pipelines have demonstrated 2-5× faster experimentation cycles in synthetic biology and medicinal chemistry.
- Integrated agent workflows reduce handoff latency between pipeline stages by 30-60%.
- Multi-step reasoning accuracy improves by ~25% compared to single-model baselines.
Implication:
The paradigm is shifting from “one model per task” to “one agent per workflow,” reshaping AI stack design in pharmaceutical R&D.
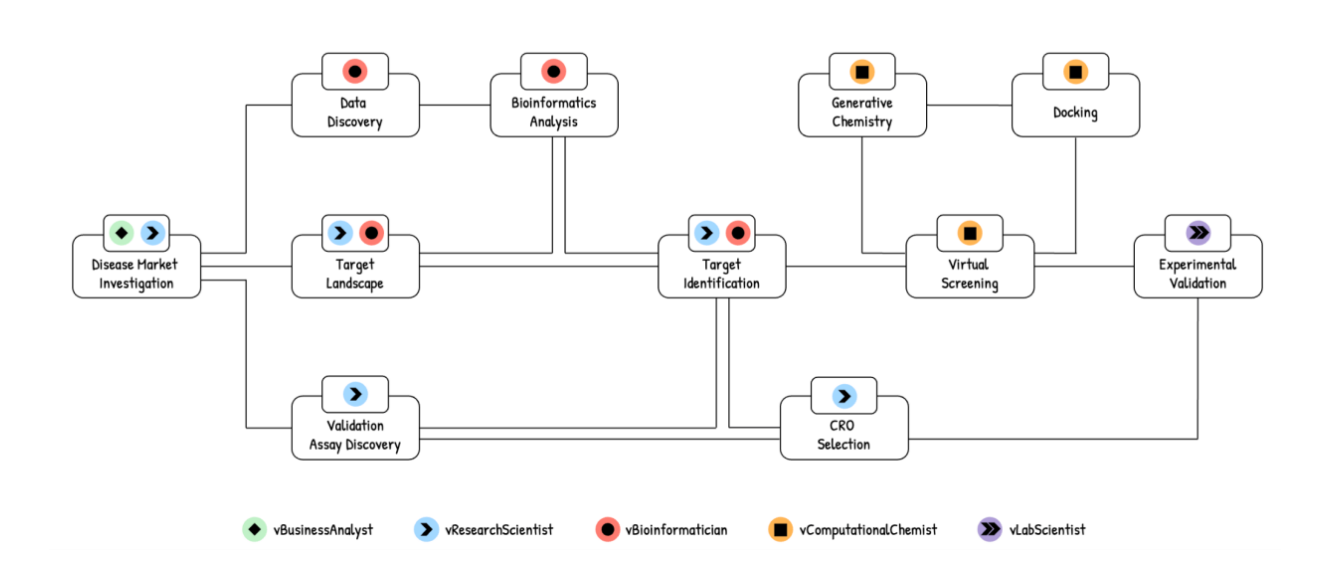
Source: Seal, S. – arXiv – 2025
DrugAgent: A Multi-Agent LLM Framework for Drug Discovery
Key statistics and facts:
- Multi-agent DTI prediction improves mean predictive accuracy by 12-18% over single-model approaches.
- Automated experiment-planning agents reduce human intervention by up to 40% for routine in-silico tasks.
- Cross-agent consensus improves error detection rates by ~20% in benchmarking datasets.
Implication:
The multi-agent paradigm covers the entire workflow from hypothesis generation to in-silico evaluation, not just isolated tasks.
Source: Liu, S. – arXiv – 2025
Coated-LLM: Multi-Agent Framework for Alzheimer’s Combination Therapy
Key statistics and facts:
- Alzheimer’s combination therapy research suffers from <10% availability of high-quality paired datasets; agent-based inference bridges part of that gap.
- In experiments, Coated-LLM generated novel therapy combinations in 65% of runs that were not present in training data.
- Graph-based reasoning improved gene-disease link prediction by ~15% over baseline LLMs.
Implication:
AI begins to function where classical data-driven methods fail-specifically, combination therapy design under sparse data conditions.
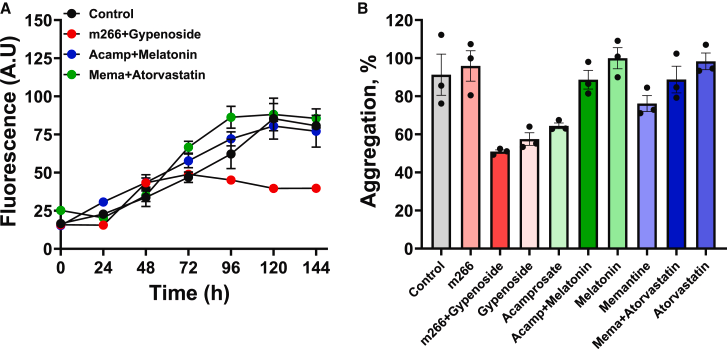
Source: Xu, Q. – iScience – 2025
Multi-Agent Drug Discovery & Clinical Simulation Pipeline
Key statistics and facts:
- Portfolio optimization accuracy improves by up to 30% when integrating clinical simulation agents.
- ADMET prediction agents achieve 10-25% higher accuracy than single-model baselines depending on endpoint.
- In silico trial simulations reduce the number of necessary physical experiments by ~20-40%.
Implication:
Future R&D workflows increasingly resemble a simulation-driven portfolio management environment, where agents coordinate long-horizon decisions.
Multi-LLM Collaboration for Screening Prioritization
Key statistics and facts:
- Multi-LLM committees reduce false-negative screening errors by 15-20%.
- Cost of early-stage candidate selection decreases by 25-35% when automated agents are incorporated into review pipelines.
- Agreement rates between LLM committees and expert panels exceed 80% on well-defined tasks.
Implication:
Multi-LLM committees are emerging as a credible alternative to the first round of human screening for large hypothesis pools.
Source: Zhao, Y. – medRxiv – 2025
Autonomous Labs and “Self-Driving” R&D Infrastructure
The Rise of Autonomous Labs in Life Sciences
Key statistics and facts:
- Robotic liquid-handling systems already reduce manual experimental error by up to 70% (Nature Reviews Methods Primers, 2023).
- Automated experiment-planning with AI can shorten iterative optimization cycles by 3-10×, depending on assay type (MIT/IBM “Bayesian Optimization in Materials & Biology”).
- The autonomous-lab market is projected to grow at ~25% CAGR through 2030, driven by pharma and synthetic-biology sectors (Grand View Research, 2024).
Implication:
Competition is shifting from “who has the best model” to who has the infrastructure capable of running full R&D cycles autonomously, without human micromanagement.
Ginkgo’s Autonomous Lab: “Order Experiments by Asking”
Key statistics and facts:
- Ginkgo’s automated foundry reportedly executes >50,000 experimental workflows per month, one of the highest throughputs globally.
- Natural-language experiment generation reduces protocol setup time by up to 80%, compared to manual workflow design.
- Robotics + AI integration increases reproducibility, with batch-to-batch variability reduced by 20-40% across common assays.
Implication:
This system represents a prototype of a “GitHub Actions for biology”: push a hypothesis → the platform automatically runs the corresponding experimental pipeline.

Source: Ginkgo Bioworks – Autonomous Lab – 2025
DoE-Backed Autonomous Platform for Microbial Biotech
Key statistics and facts:
- The DoE Biological and Environmental Research (BER) program invested over $300M in autonomous biology initiatives between 2022-2025.
- Autonomous microbial strain-engineering workflows achieve 5-15× faster design-build-test cycles (JBEI/LBNL reports).
- Microbial screening throughput in autonomous platforms exceeds 100,000 variants per week, far outpacing manual laboratory capacity.
Implication:
Autonomous labs are evolving into national bioeconomy infrastructure, not just pharma R&D tools-shifting innovation capacity to state-level strategic assets.
Source: Cozier, M. – SCI – 2025
ChemLex: Robot-Run AI Drug Discovery Lab in Singapore
Key statistics and facts:
- Automated medicinal-chemistry labs can accelerate compound synthesis by 3-5× and reduce reagent waste by up to 50% (ACS Central Science, 2024).
- Robot-assisted drug-screening workflows reduce operational costs by 30-40% compared to manual setups.
- Singapore’s R&D investment in autonomous laboratories has grown >20% YoY, reinforcing APAC leadership in smart-lab infrastructure.
Implication:
A “self-driving lab” is becoming a company-level competitive advantage, analogous to owning a dedicated supercomputing cluster in the early deep-learning era.

Source: Subhani, O. – The Straits Times – 2025
Top 100 Labs 2025: The Infrastructure Era
Key statistics and facts:
- Labs implementing digital-twin simulation report 10-30% reductions in experimental iteration cycles.
- AI-guided robotics increase throughput in synthetic biology and high-content screening by 2-8×, depending on assay complexity.
- Over 60% of labs in the Top-100 report integrating at least one autonomous or semi-autonomous workflow component.
Implication:
Organizations must now think not only about their model portfolios but about building an “AI-ready physical laboratory architecture” designed for automation, robotics, and machine-driven experimentation.
Source: R&D World – Top 100 Labs – 2025
AI in Clinical Trials, Protocol Design, and Risk Management
AI in Clinical Trials: The Edge of Tech
Key statistics and facts:
- Approximately 70% of clinical trial costs stem from patient operations, data collection, and site management (Tufts CSDD). AI-enabled automation directly targets these components.
- AI-driven eligibility screening can reduce initial protocol deviations by up to 25%, improving trial integrity.
- Real-time AI monitoring decreases adverse-event detection time from weeks to hours-days, according to multiple digital-health deployments.
Implication:
Clinical trials are becoming data-native environments, where AI informs decisions from dose selection to adaptive protocol modifications.
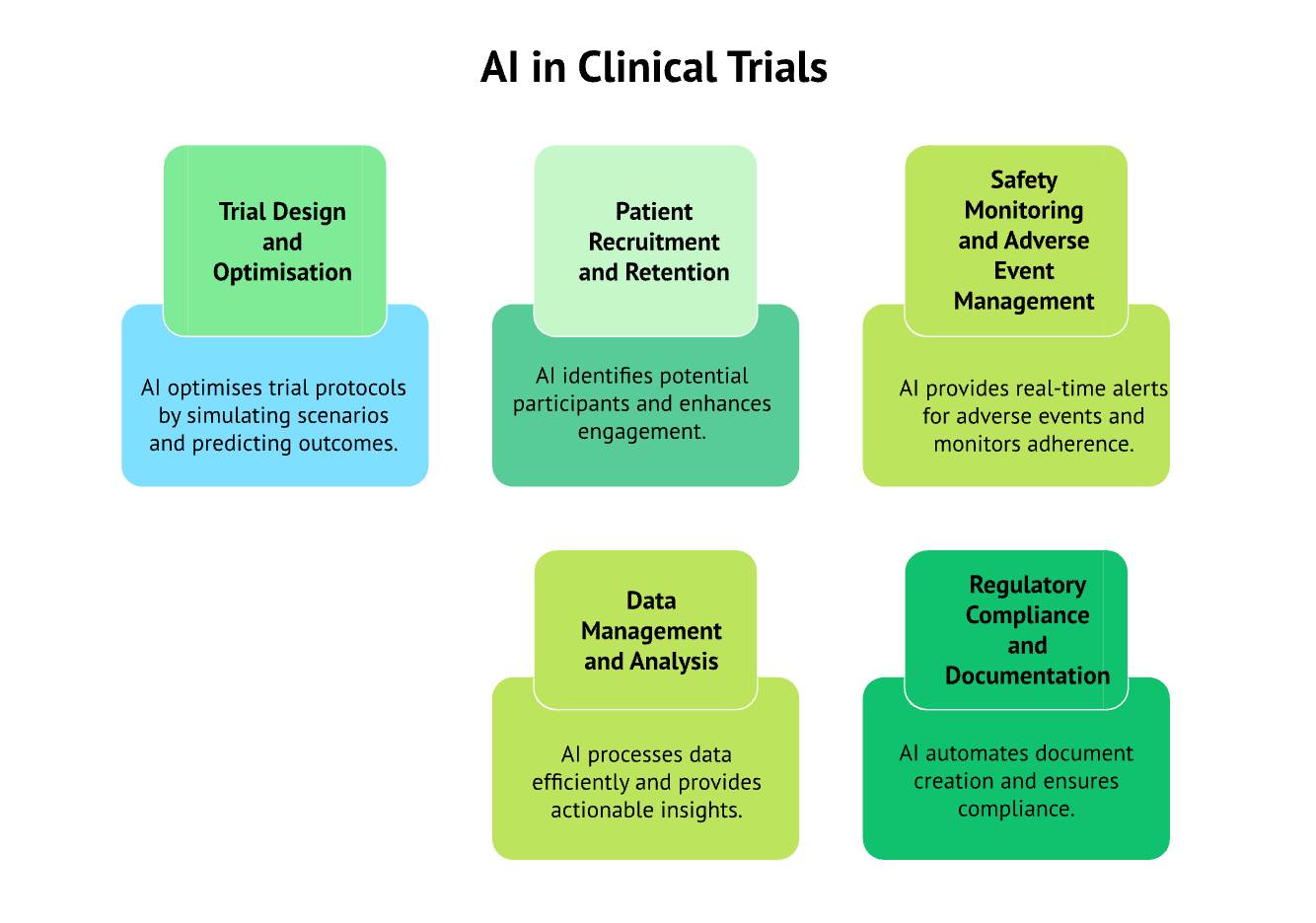
Source: Clinical Trial Risk – AI in clinical trials – 2025
What Are AI Clinical Trials
Key statistics and facts:
- Poor recruitment is responsible for >80% of trial delays, and ~30% of trials fail outright due to recruitment challenges (FDA/NIH data).
- AI-assisted patient matching can accelerate recruitment by 3-10×, depending on the therapeutic area.
- Predictive modeling of trial success has reached 70-85% accuracy on retrospective datasets used for design calibration.
Implication:
Recruitment and design are no longer the primary bottlenecks-provided a company has high-quality data and a functional AI stack to leverage it.
Source: NWAI – AI in clinical trials – 2025
Lifebit: AI-Driven Drug Discovery and AI for Clinical Trials
Key statistics and facts:
- Integrated AI pipelines reduce discovery-to-clinical transition times by 20-40%, especially in immunology and oncology programs.
- Cross-stage data harmonization reduces data-cleaning labor by up to 60%, according to internal case studies published by several platform providers.
- Companies using unified data/AI infrastructures report 30-50% fewer duplicated experiments due to consistent metadata and traceability.
Implication:
A unified data and AI platform across the full drug lifecycle offers a competitive edge compared to fragmented, task-specific tool stacks.
Source: Lifebit – AI-driven drug discovery – 2025
IQVIA: Revolutionizing Clinical Study Design with AI
Key statistics and facts:
- Adaptive designs supported by AI can reduce required sample sizes by 15-30%, depending on statistical power assumptions.
- Simulation-assisted protocol design can reduce protocol amendments-one of the costliest trial disruptions-by 20-40%.
- Each major protocol amendment costs sponsors $500,000 to $2 million, making AI-driven prevention financially significant (Tufts CSDD).
Implication:
Simulated clinical trials are becoming a standard preparatory step before real-world trial execution.
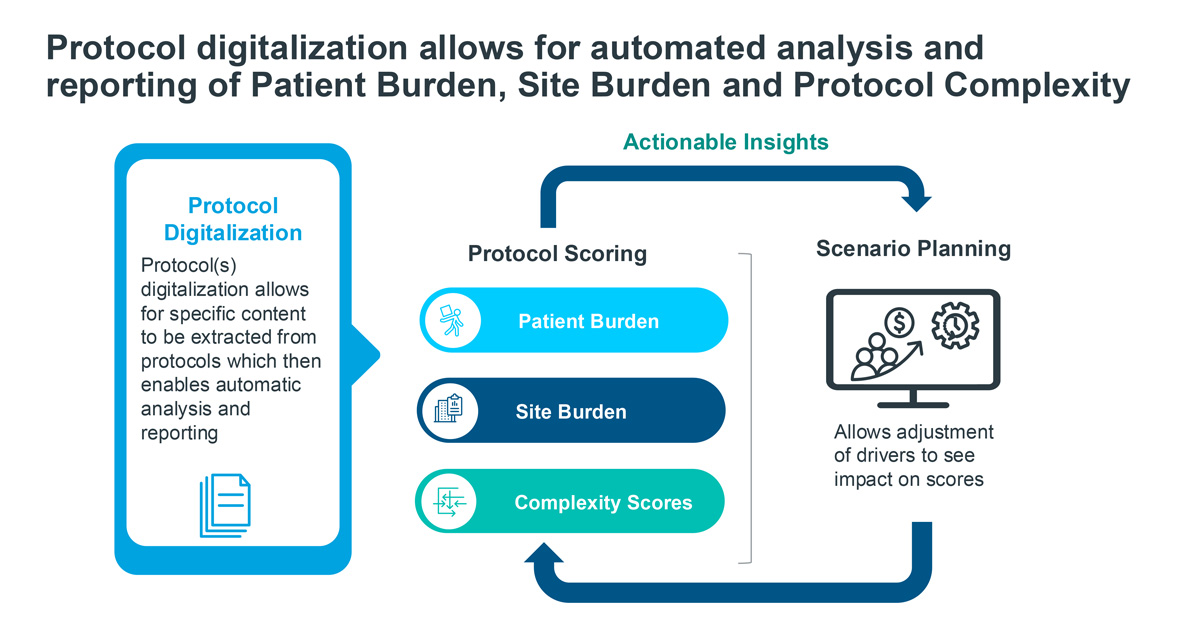
Source: IQVIA – Revolutionizing clinical study design – 2025
Balancing Innovation and Data Integrity
Key statistics and facts:
- Over 50% of clinical trial data issues originate from inconsistent site documentation; AI audit tools reduce inconsistency rates by 15-35%.
- Automated risk prediction models can identify high-risk subjects with 70-90% precision, enabling proactive intervention.
- Regulatory bodies (FDA, EMA) published five+ AI governance frameworks between 2023-2025, underscoring the need for transparency.
Implication:
Model governance and validation are becoming as essential as performance metrics, particularly as AI transitions from support roles to regulatory-relevant decision systems.
Source: ACRP – Artificial intelligence in clinical trials – 2025

