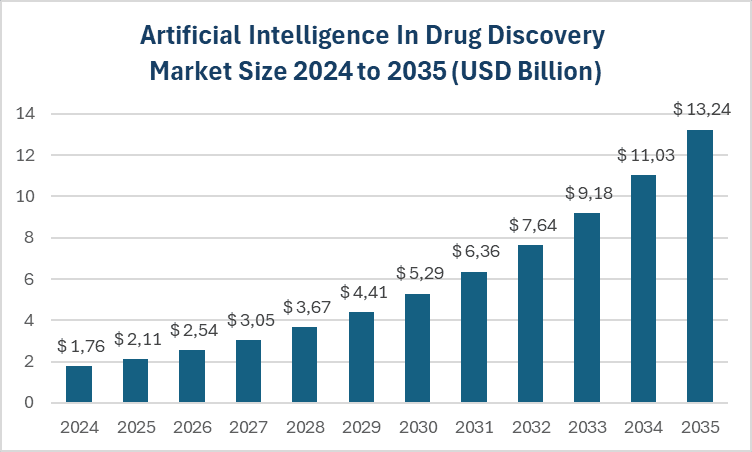
Following the overall trend across the biotechnology sector, AI presence in drug development is already significant and continues to grow. This market is valued at USD 1.76 billion in 2024 and is projected to reach a value of USD 13,24 billion by 2035, with a CAGR of 20.15% (Vantage Market Research).
Artificial intelligence plays a key role in determining the future of drug development, making the process faster, more efficient, cheaper, and addressing unmet medical needs. AI-driven tools capable of optimizing every stage of the pharmaceutical pipeline from target identification and drug screening to design, clinical trials, and manufacturing. What may be most valuable is that they also simplify drug repurposing.
This year for the industry was remarkable with breakthroughs, but some challenges are still unsolved.
A real-world example of this approach can be seen in our AI-Driven Drug Discovery case, where we developed predictive molecular models to streamline target selection and accelerate hit-to-lead optimization.
Read the Full CaseSteps of Drug Discovery
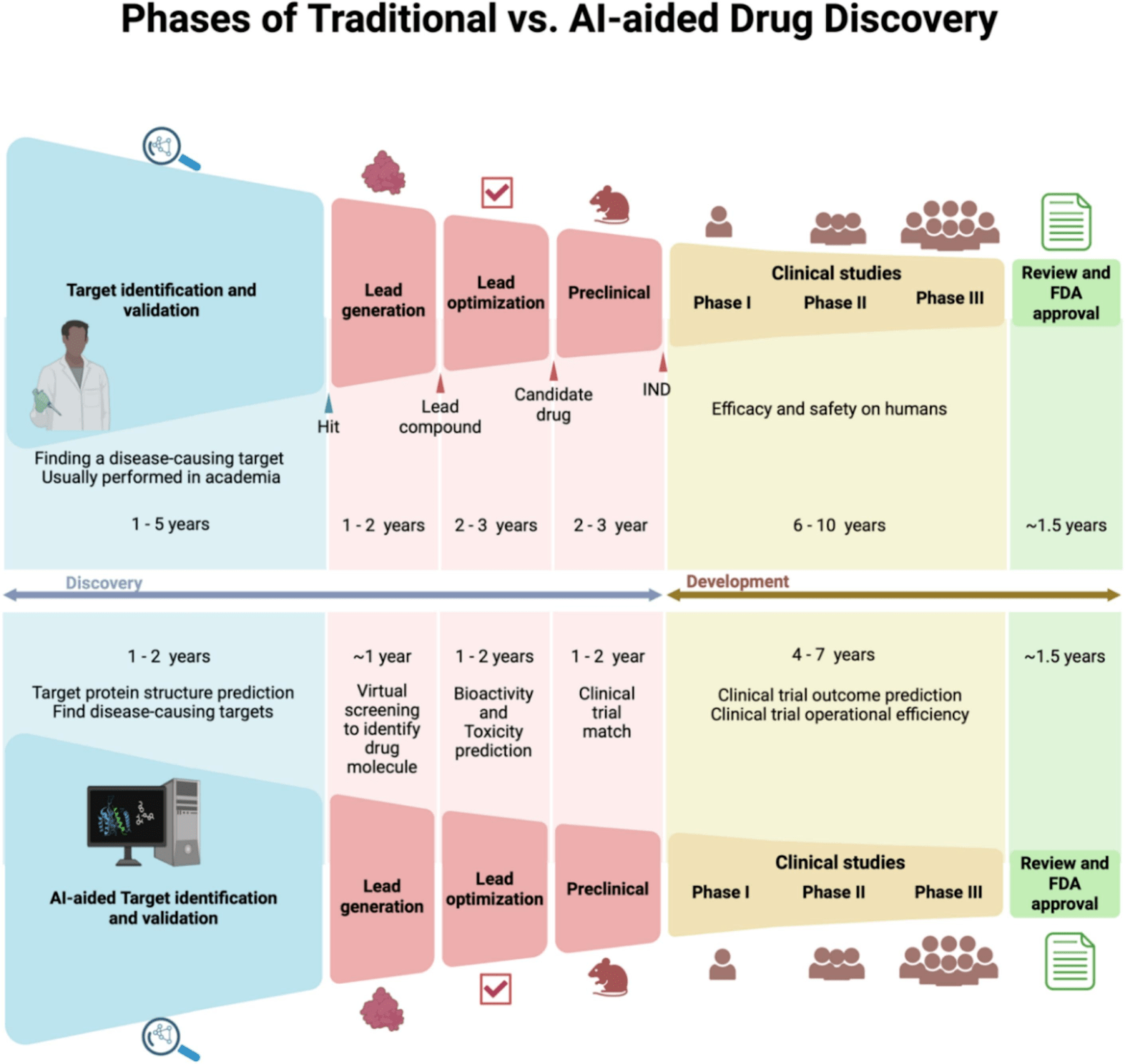
The pharmaceutical industry is constantly evolving, with drug development being one of its most dynamic and challenging areas. Traditionally, this process has been lengthy, complex, and costly, with high risks and uncertainties. However, the integration of artificial intelligence (AI) into drug development is transforming the landscape. AI’s ability to analyze vast datasets, predict outcomes, and streamline processes is accelerating the pace of drug discovery, enhancing the precision of predictions, and significantly reducing development costs.
By automating complex tasks and providing deeper insights, AI enables more effective treatments to reach the market faster. Artificial Intelligence not only improves drug discovery directly, but it also helps with research along the way.
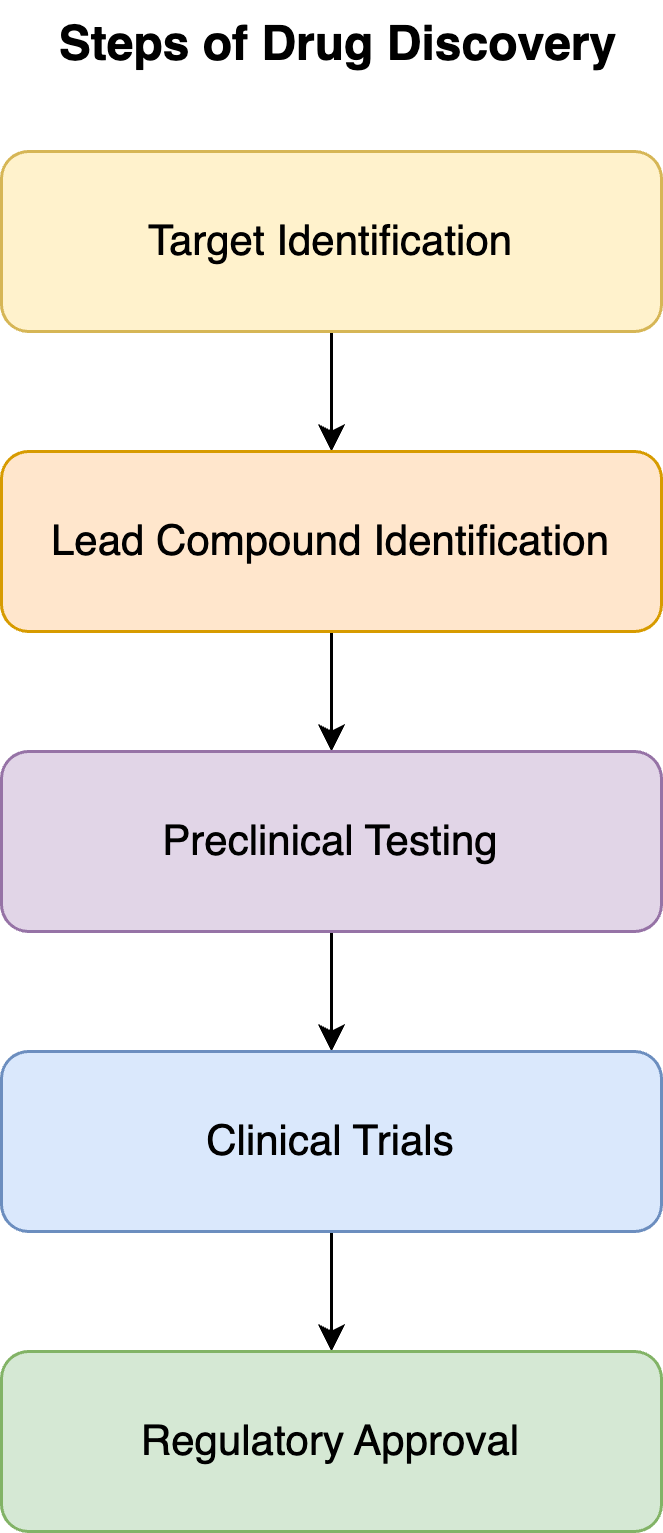
The drug discovery process is a multi-step journey that involves rigorous research, testing, and validation. The key stages include:
- Target Identification: Identifying the biological target associated with a disease (e.g. type 2 diabetes/obesity disease and glucagon-like peptide-1 receptor target).
- Lead Compound Identification: Discovering a chemical compound that interacts with the target (e.g. semaglutide).
- Lead Compound Assessment and Optimization: scoring the lead compound and refining it to enhance its efficacy and minimize toxicity.
- Preclinical Testing: Conducting lab-based studies to evaluate the safety and efficacy of the compound.
- Clinical Trials: Testing the drug in humans to ensure its safety and effectiveness across multiple phases.
- Regulatory Approval: Submitting the drug for approval from regulatory bodies before it can be marketed.
Each of these steps in the drug discovery process requires rigorous data collection, thorough analysis, and precise interpretation to ensure successful outcomes. Traditionally, these tasks were handled manually, often leading to significant delays and potential errors. However, with the use of AI, we are now able to automate and streamline critical aspects of this process.
Specifically, AI can also play a pivotal role in enhancing the efficiency and accuracy of compound stability assessments and toxicity evaluations, such as hERG blocker classification—two crucial steps that can significantly impact the speed and success of drug development.
How AI Transformed Drug Discovery
It may sound surprising, but in fact, AI isn’t something new in the pharmaceutical field, as it has been used for nearly three decades.
However, the implementation of artificial intelligence has matured from a conceptual phase to a practical application relatively recently, starting around 2018. Over this period, AI has gone through periods of both rapid progress and setbacks (Fu et al., 2025).
In recent years, the pharmaceutical industry has achieved minimal success in delivering novel drugs to the market despite the high investments. Out of 5,000 compounds at the preclinical stage, only five progress to clinical trials, and only one may be approved (Jarallah et al. 2025). Then the COVID-19 pandemic posed a serious challenge for the industry.
This situation required fundamental changes. Therefore, it is not surprising that the implementation of artificial intelligence has become widespread in pharma.
Speed, efficiency, accuracy, cost reduction – what else can be provided by AI-driven technologies?
Let’s take a closer look at innovations that were enabled by artificial intelligence and their role in defeating current challenges.
Target identification and validation:
- Integration of diverse and complex biological datasets;
- Data-driven identification of causal disease drivers and biomarkers;
- Exploration of novel targets, even for diseases that were previously untreatable or poorly managed;
- Prediction of drug-target interactions, either by modeling protein 3D structures from sequences or by profiling interactions at a genome-wide scale;
- Identification of homologous genes and proteins through sequence similarity searches;
- Effective prioritization of therapeutic targets;
- Reducing the reliance on experimental strategies (Jarallah et al. 2025).
Drug screening:
- Implementation of ML/DL to rapidly evaluate millions of compounds against biological targets;
- Prediction of active compounds from known ligands without requiring protein structures;
- Improved docking accuracy and binding affinity prediction;
- Generative AI proposes novel compounds to test;
- Early filtration of unsuitable molecules;
- Integration of multiple criteria (bioactivity, selectivity, ADMET) for efficient hit prioritization (Jarallah et al. 2025).
Drug design:
- De novo drug design from scratch using computational algorithms;
- Diverse dataset integration (structural, pharmacokinetic, and bioactivity data), their filtration, and classification to optimize molecules for desired traits;
- Integration of chemical rules to ensure that novel molecules are synthetically accessible;
- Prediction of toxicity, pharmacokinetics, ADMET, solubility prediction, skin permeability, and physicochemical properties(Jarallah et al. 2025).
Drugs Manufacturing
An AI-driven automation system can improve the quality of the drugs and lower operational costs by:
- Maintaining the right conditions for sensitive products;
- Reduce errors;
- Boost efficiency;
- Optimize the entire supply chain;
- Predict when equipment needs maintenance;
- Simulate the entire manufacturing process in real time to identify potential inefficiencies (Coherent Solutions).
Clinical trials:
- Prediction of clinical trial outcomes by analyzing drug response, toxicity, and side effects;
- Optimization of clinical trial design;
- Sequencing data and histological information to uncover drivers of disease and drug response;
- Data-powered patient recruitment;
- Improving patient stratification: links genetic, molecular, and clinical data to classify patients into subgroups with different responses;
- Monitoring that patients take medications correctly;
- Virtual and in silico trials reduce the number of required participants and improve statistical power;
- Enhances decision-making and supports precision medicine strategies by integrating diverse datasets (Fu et al., 2025).
Drug Repurposing
Rather than “reinventing the wheel”, it is often more practical to find new therapeutic uses for existing drugs.
AI facilitates this by predicting novel drug–disease relationships through the integration of chemical structures, biological activity, and disease phenotypes. It can also leverage gene expression data to uncover mechanism-based repurposing opportunities.
Graph neural networks model drug–target, protein–protein, and disease–gene interactions, which, along with integration of multi-omics and patient data, enable personalized drug repurposing for rare and complex diseases (Jarallah et al. 2025).
Current backlogs and future
Despite the promising opportunities offered by AI, unsolved challenges:
- Performance can be affected if the data is complex, variable or has low quality;
- Proposed molecules may not always be viable for synthesis or further development;
- There are legal and ethical issues with intellectual property rights and patient privacy;
- Many models operate as “black boxes”, creating challenges for approval and interpretability;
- Al models often struggle with generalization and robustness, including overfitting to specific datasets, vulnerability to adversarial attacks, and limited transferability across studies.
- Implementation of advanced AI systems is a resource-intensive process;
- Algorithms’ bias and false positives/negatives distort the results;
- Experimental validation remains a critical step (Kokudeva et al.,2024; Fu et al., 2025; Jarallah et al., 2025);
To overcome these concerns, it’s necessary to improve algorithms, integrate diverse datasets, provide systematic organization of AI technologies, and implement security measures (Jarallah et al. 2025). Further development will rely on a cycle of “big data→more precise models→better drugs→more and better data” (Fu et al., 2025).
On the other hand, success will also depend on specialists from different fields. Multidisciplinary education and collaboration among researchers, clinical experts, engineers, and data managers have now become crucial (Kokudeva et al., 2024).
The potential of Al-based methods to address unmet medical needs and enhance the efficiency of drug development is already demonstrated by successful case studies. However, the widespread adoption and integration of such technologies takes time. Before AI- and data-driven pharmaceutical models can fully realize their potential, further development and resolution of current challenges are necessary.

Get a one-on-one consultation with Ivan Izonin, PhD, Scientific Advisor in Artificial Intelligence, and learn how to apply advanced AI methods across your drug discovery pipeline – from data analysis to clinically relevant insights.
Book a ConsultationEnhancing Compound Stability Assessment with AI
In the drug discovery process, assessing the metabolic stability of a compound is crucial. Metabolic stability refers to how long a drug remains intact before it is metabolized by the body, which directly impacts its efficacy and safety. Understanding the stability of a compound is vital in drug development because it directly impacts how long a drug will be effective in the body.
Traditionally, this assessment involves complex laboratory procedures and the analysis of large datasets to determine the compound’s stability under various conditions.
Our AI-powered Metabolic Stability Estimator simplifies this assessment. By analyzing the chemical structure of a compound, it quickly predicts whether a drug candidate is likely to remain stable under various conditions or degrade too quickly.
The business value here is clear: faster and more reliable stability assessments mean you can move forward with promising drug candidates more quickly, saving valuable time and resources in the development process.
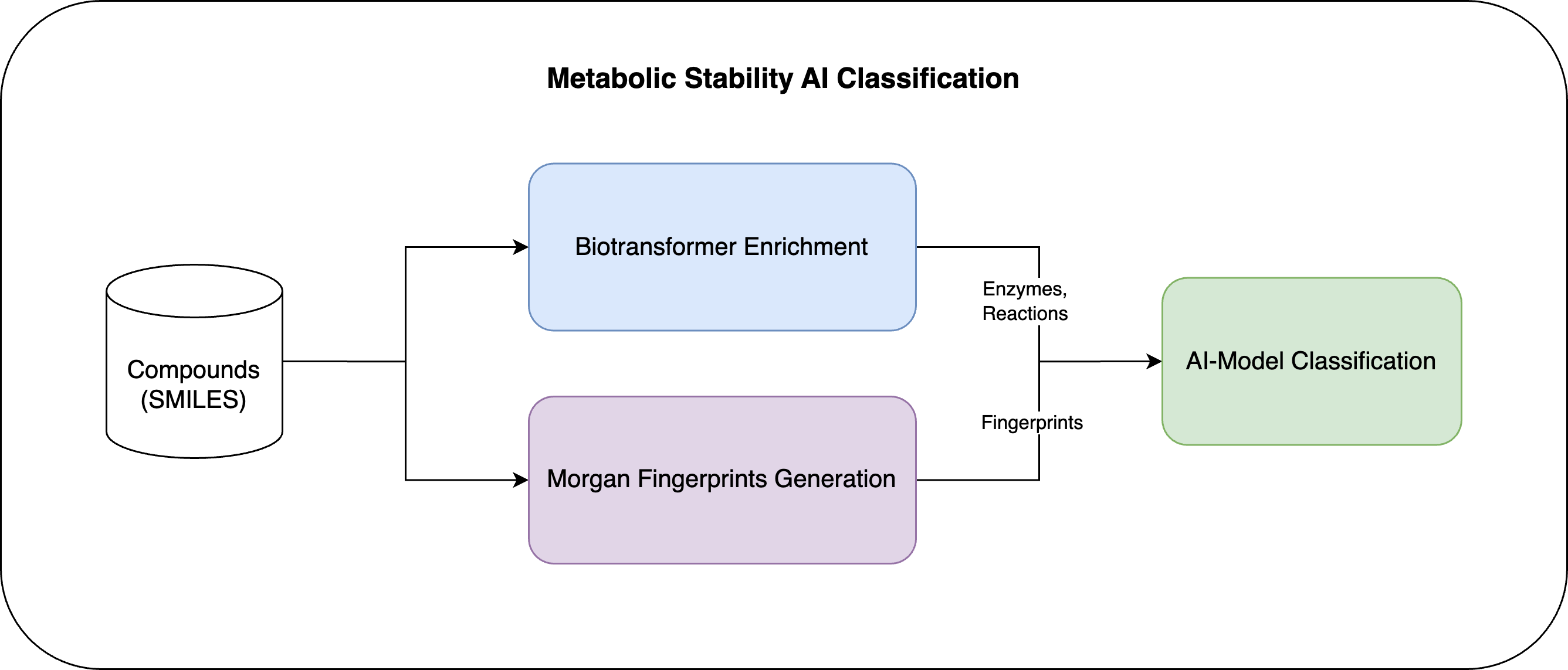
From a technical standpoint, our AI-powered Metabolic Stability Estimator works by first computing enzymes, reactions, and fingerprints for the compound utilizing the Biotransformer toolkit and Morgan Fingerprint algorithm. These features are then combined and transformed to serve as input to the AI model for predicting metabolic stability.
Enhancing Compound Toxicity Assessment with AI
One of the biggest concerns in drug development is ensuring that a new drug won’t cause harmful side effects, particularly those related to heart health. Specifically, it’s crucial to determine whether a compound might interfere with the heart’s electrical activity, which is often assessed through hERG (human Ether-a-go-go-Related Gene) testing. Compounds that block the hERG channel can lead to serious cardiac side effects, such as arrhythmias, making this a critical checkpoint in the drug development process.
Traditionally, hERG toxicity is assessed using a combination of laboratory testing and computational modeling. In the lab, researchers use patch-clamp assays, which are highly specialized experiments that measure the ionic currents in heart cells. This method, while accurate, is both time-consuming and costly. It requires access to expensive equipment, skilled technicians, and extended periods of time to obtain results.
Our AI-powered hERG Blocker Classifier addresses these challenges head-on. By leveraging artificial intelligence algorithms, the tool quickly analyzes a compound’s chemical structure and predicts its potential to block the hERG channel. This AI tool is built on large datasets that include both toxic and non-toxic compounds, enabling it to make accurate predictions even with novel compounds that haven’t been extensively studied before.
The ability to make such assessments quickly and accurately means that your development team can focus resources on the most promising candidates, thereby increasing the likelihood of success in later stages of development.
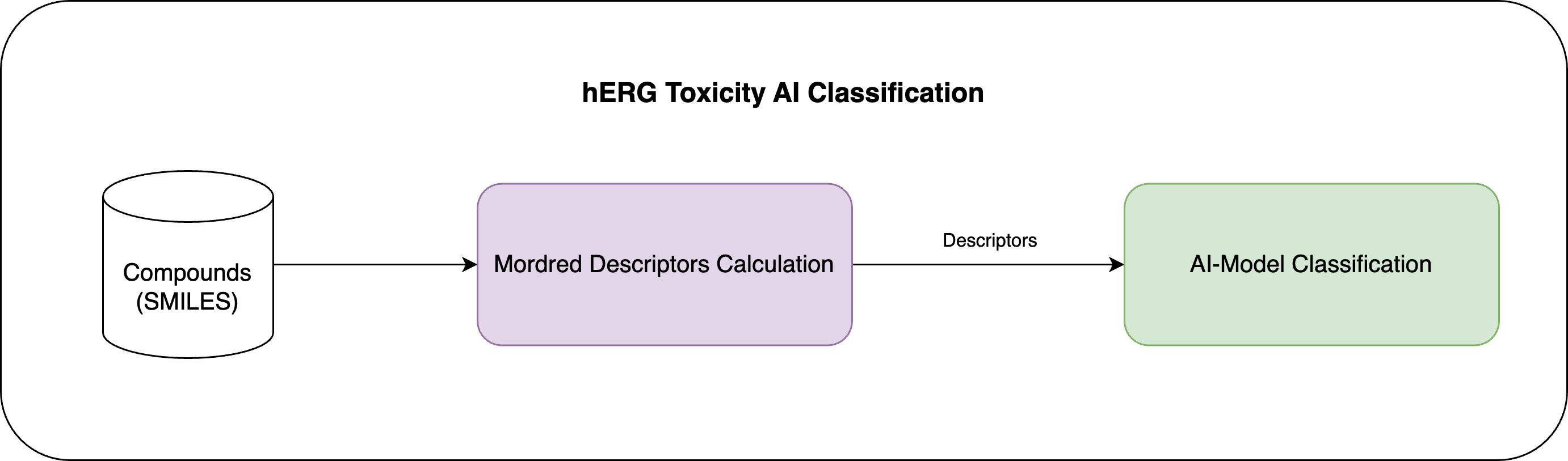
From a technical standpoint, our AI-powered hERG Blocker Classifier works by first computing Mordred Descriptors for the compound. These features are then transformed to serve as input to the AI model for predicting hERG toxicity assessment.
Latest Breakthroughs in AI for Drug Discovery: What Will Make 2025 Memorable
Despite substantial AI-enabled generative chemistry, few novel AI-discovered or AI-designed drugs have advanced to clinical trials. However, Insilico Medicine’s small-molecule TNIK inhibitor successfully completed a Phase 2a trial. While further investigation is needed, the study confirmed not only the compound’s therapeutic potential but also alleviated doubts about the feasibility of using artificial intelligence in drug discovery (Xu et al., 2025).
Another game-changing technology that was presented this year is MULTICOM4 – a tool for structural biology that overcame long-lasting challenges with modeling large assemblies and handling protein complexes, especially with poor MSAs or unknown subunit counts. It was enabled by combining AlphaFold’s models and enhancing them with additional ML-driven components (Liu et al., 2025).
Artificial intelligence applications have expanded to more complex modalities, such as macrocycles, ADCs, and RNA-targeting therapies. Additionally, numerous AI-driven platforms for diverse tasks in drug design and development have been launched or improved.
Key Use Cases of AI for Drug Discovery by Blackthorn AI
AI-Driven Molecule Generation
The goal of this project was to identify and prioritize novel small molecules for the dermatology field.
The created pipeline allows rapid iteration, incorporates biological constraints, and reduces reliance on trial-and-error lab synthesis. It’s based on a combination of generative AI, pathway graphs, and omics data.
Multi-omics AI data platform
The aim was to refine a platform for drug, indication, target discovery, and repurposing, while building a product that functions with minimal oversight.
To address this challenge, the team developed a multi-omics AI platform for generating drug candidates with specific physicochemical and biological properties, including logP, logS, pKa, BBBP, binding affinity, metabolic stability, and toxicity.
This system consists of:
- Web portal;
- Data processing pipeline;
- A multi-omics data lakehouse;
- Knowledge graph with Gen AI.
In just six months, the team achieved:
- 36% faster time to insight from multi-omics datasets;
- Platform capability to handle 5× more users without team expansion;
- Rapid deployment of future modules, as 75% of the codebase is reusable;
- 99.5% uptime thanks to GCP infrastructure.
You can find more information about these projects and view others in Case Studies section of our website.
Conclusion
AI has arrived as a timely solution for the pharmaceutical industry, addressing challenges of cost, efficiency, and low success rates in traditional drug discovery.
Numerous AI tools have been developed to enhance all stages of drug discovery and development and to make drug repurposing more efficient. They enable addressing unmet needs in personalized medicine and the treatment of rare diseases. And finally, the industry has received real validation, as AI-enabled drugs have successfully advanced to clinical trials.
Despite the progress, there are still many limitations that require improvement of data quality and diversity, the resolution of the “black box” problem, and the establishment of clear ethical and legal standards.
Integrating AI into drug discovery is not just a technological upgrade – it’s a strategic move to enhance efficiency, accuracy, and speed in developing new treatments. By automating tasks like metabolic stability and hERG toxicity assessments, AI tools significantly reduce the time and resources needed to bring a drug to market.
Key Benefits
- Metabolic Stability Estimator
- Speed: Cuts assessment time from weeks to hours.
- Cost: Lowers expenses by reducing lab work.
- Impact: Enables faster, more informed choices.
- hERG Blocker Classifier
- Speed: Reduces toxicity classification from weeks to hours.
- Precision: Delivers reliable early-stage insights.
- Cost: Automates and lowers assessment costs.
Adopting AI in your drug discovery pipeline gives you a crucial edge in a competitive industry, ensuring your R&D is both innovative and cost-effective. As the pharmaceutical industry evolves, integrating AI is essential for bringing life-saving drugs to market faster and more efficiently.
To sum up, artificial intelligence has already confirmed its potential in drug discovery and development. In the coming years, AI-driven technologies will certainly have an important role, as they can accelerate the whole pharmaceutical workflow, reduce expenses, and deliver safer, more effective medicines.If you’re interested in AI-driven drug development, feel free to reach out to us at blackthorn.ai. You can also book a training session or consultation with Alex Gurbych, PhD, CEO.